To mix or not to mix? Nanoalloys at or out of equilibrium
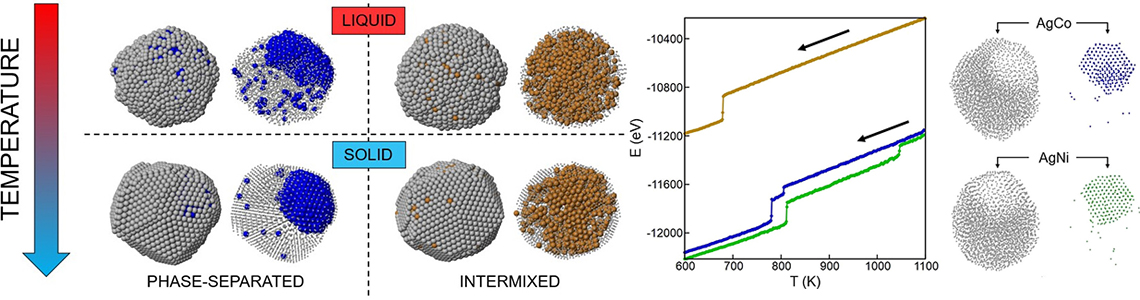
The extraordinary properties of metal nanoparticles can be further enhanced and engineered by mixing two or more metals: this is the realm of nanoalloys. The structure and chemical ordering of nanoalloys determines their technological applicability, e.g., in catalysis. Computer simulations allowed to describe the production of bimetallic nanoparticles by cooling down nanodroplets of silver alloys until they solidification. Nickel and cobalt do not mix with silver even in the liquid state and remain separated in the solid. This behaviour is in line with equilibrium considerations, i.e., does not depend on the specific production process we simulate. Copper, on the other hand, mixes with silver in the liquid state but is expected to phase-separate in the solid. However, during the cooling process, we observed that AgCu nanoparticles remained trapped in a mixed state which is significantly different from the equilibrium one. Simulations allowed us to observe the detailed mechanisms of solidification like in an atomic microscope, revealing that AgCo and AgNi solidify in two steps, starting from the cobalt/nickel part which facilitates the solidification of the silver shell; solidification of AgCu, instead, happens homogeneously in a single step from inside the intermixed nanodrop.
More info:
https://pubs.acs.org/doi/10.1021/acsnano.2c09741